- :
- Unit Price:
- Quantity:

-
Code:002216-002
- ESTRIEL Tab. 1mg : 100 tablets
- $48.49
Up to 1 pieces per person
10% discount for total amount over $100
shipping schedule : 5 to 10 working days after payment.
warehouse:Japan1
Manufacturer :Mochida Pharmaceutical
Brand :Mochida Pharmaceutical
JAN Code:----
-
Description
-
This medicine is one (follicle hormone) of female hormone. It controls hormone balance and suppresses climacteric symptom (hot flush, headache, sleeplessness, palpitation, etc.) or vaginal inflammation by supplying lacking female hormone. It also controls the balance between bone formation and bone resorption, and it inhibits the decrease of bone mineral density.
It is usually used to treat menopausal disorder, vaginitis (senile, children and non-specific), cervicitis and cervicovaginal erosion and senile osteoporosis.
-
Presentation
-
100 tablets
-
Feature
-
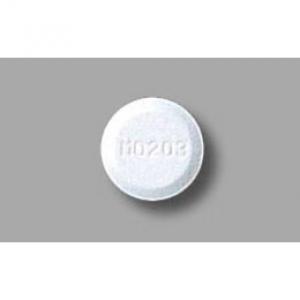
white tablet, diameter: 8.0 mm, thickness: 3.0 mm
-
Active Ingredients
-
Estriol 1mg
-
Effect/Efficacy
-
This medicine is one (follicle hormone) of female hormone. It controls hormone balance and suppresses climacteric symptom (hot flush, headache, sleeplessness, palpitation, etc.) or vaginal inflammation by supplying lacking female hormone. It also controls the balance between bone formation and bone resorption, and it inhibits the decrease of bone mineral density.
It is usually used to treat menopausal disorder, vaginitis (senile, children and non-specific), cervicitis and cervicovaginal erosion and senile osteoporosis.
-
Usage/Dosage
-
Menopausal disorder, vaginitis (senile, children and non-specific), cervicitis and cervicovaginal erosion: In general, for adults, take 0.1 to 1.0 mg of the active ingredient at a time, once to twice a day. The dosage may be adjusted according to the disease, age or symptoms. This preparation contains 1 mg of the active ingredient in a tablet.
Senile osteoporosis: In general, for adults, take 1 tablet (1.0 mg of the active ingredient) at a time twice a day. The dosage may be adjusted according to the symptoms.
In any case, strictly follow the instructions.
If you miss a dose, take a dose as soon as possible when you noticed. If it is almost time for the next dose, skip the missed dose and follow your regular dosing schedule. You should never take two doses at one time.
If you accidentally take more than your prescribed dose, consult with your doctor or pharmacist.
Do not stop taking this medicine unless your doctor instructs you to do so.
-
Cautions
-
If you have previously experienced any allergic reactions (itch, rash, etc.) to any medicines.
If you have breast cancer (including history or doubt), endometrial cancer (including doubt), endometrial hyperplasia (under or unfinished treatment), thrombophlebitis/pulmonary embolism/coronary heart disease/stroke (including history), liver disorder, unexplained genital bleeding.
If you are pregnant, possibly pregnant or breastfeeding.
If you are taking any other medicinal products. (Some medicines may interact to enhance or diminish medicinal effects. Beware of over-the-counter medicines and dietary supplements as well as other prescription medicines.)
Keep out of the reach of children. Store away from direct sunlight, heat and moisture.
Discard the remainder. Do not store them.
-
Contraindication
-
The most commonly reported adverse reactions include nausea, loss of appetite, irregular bleeding, breast tension feeling, rash and itch. If any of these symptoms occur, consult with your doctor or pharmacist.
The symptoms described below are rarely seen as initial symptoms of the adverse reactions indicated in brackets. If any of these symptoms occur, stop taking this medicine and see your doctor immediately.
in case of long-term use, lower limb pain/edema, sudden breathing difficulty, severe headache [thrombosis]
The above symptoms do not describe all the adverse reactions to this medicine. Consult with your doctor or pharmacist if you notice any symptoms of concern other than those listed above.