- :
- Unit Price:
- Quantity:
-
Code:705167-001
- Regpara Tablets 75mg:20 tablets
- $607.49
Up to 2 pieces per person
10% discount for total amount over $100
shipping schedule : 5 to 10 working days after payment
warehouse:Japan1
Manufacturer :Kyowa Kirin Co., Ltd.
Brand :Kyowa Kirin Co., Ltd.
JAN Code:----
-
Description
-
This medicine directly acts on calcium receptors on parathyroid cells to suppress synthesis and secretion of parathyroid hormone (PTH), and it consequently decreases serum PTH and serum calcium.
It is usually used in the treatment of hypercalcemia in patients with parathyroid carcinoma, and hypercalcemia in patients with primary hyperparathyroidism who are unable to undergo parathyroidectomy or who experience recurrent primary hyperparathyroidism.
-
Presentation
-
20tablets
-
Feature
-
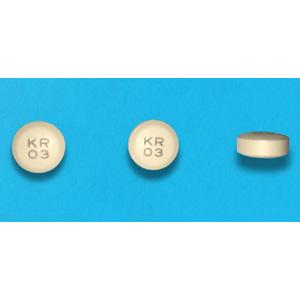
light-yellow tablet, diameter: 8 mm, thickness: 4 mm
-
Active Ingredients
-
Cinacalcet hydrochloride
-
Effect/Efficacy
-
This medicine directly acts on calcium receptors on parathyroid cells to suppress synthesis and secretion of parathyroid hormone (PTH), and it consequently decreases serum PTH and serum calcium.
It is usually used in the treatment of hypercalcemia in patients with parathyroid carcinoma, and hypercalcemia in patients with primary hyperparathyroidism who are unable to undergo parathyroidectomy or who experience recurrent primary hyperparathyroidism.
-
Usage/Dosage
-
In general, for adults, start with taking 25 mg of cinacalcet at a time, twice daily. Then the dosage may be adjusted to take 25 to 75 mg at a time, twice daily, by observing serum calcium level. Dose should be increased by 25 mg at a time, at intervals of at least two weeks. The dosage may be increased up to a maximum dose of 75 mg at a time, 3 or 4 times a day. This preparation contains 75 mg of cinacalcet in a tablet. Strictly follow the instructions.
If you miss a dose, skip the missed dose and continue your regular dosing schedule. You should never take two doses at one time.
If you accidentally take more than your prescribed dose, consult with your doctor or pharmacist.
Do not stop taking this medicine unless your doctor instructs you to do so.
-
Cautions
-
If you have previously experienced any allergic reactions (itch, rash, etc.) to any medicines.
If you have: hypocalcaemia or hepatic function disorder, seizure or a history of it.
If you are pregnant or breastfeeding.
If you are taking any other medicinal products. (Some medicines may interact to enhance or diminish medicinal effects. Beware of over-the-counter medicines and dietary supplements as well as other prescription medicines.)
Grapefruit and grapefruit juice may intensify the therapeutic effects of the medicine. Do not eat grapefruit or drink grapefruit juice while you are taking the medicine.
Keep the medicine out of the reach of children. Store it away from direct sunlight, heat and moisture.
Discard the remainder. Do not store them.
-
Contraindication
-
The most commonly reported adverse reactions include nausea, vomiting, gastroesophageal reflux disease, paresthesia, decreased body weight and decreased appetite. If any of these symptoms occur, consult with your doctor or pharmacist.
The symptoms described below are rarely seen as initial symptoms of the adverse reactions indicated in brackets. If any of these symptoms occur, stop taking this medicine and see your doctor immediately.
numbness, seizure, feeling unwell, arrhythmia, decreased blood pressure [hypocalcaemia, decreased serum calcium]
palpitation, chest discomfort, chest pain [prolonged QT interval]
vomiting of blood, nausea, gastric pain [gastrointestinal hemorrhage, gastrointestinal ulcer]
impaired mind, judgment failure, temporary loss of consciousness [decreased level of consciousness, temporary loss of consciousness]
The above symptoms do not describe all the adverse reactions to this medicine. Consult with your doctor or pharmacist if you notice any symptoms of concern other than those listed above.